Baby Chicken Pox From a Vaccinated Person Wild Strain or Not?
Varicella (chickenpox) is an acute infectious disease. It is caused by varicella-zoster virus (VZV), which is a DNA virus that is a member of the herpesvirus group. After the primary infection, VZV stays in the trunk (in the sensory nerve ganglia) every bit a latent infection. Primary infection with VZV causes varicella. Reactivation of latent infection causes canker zoster (shingles).
- Clinical Features
- Transmission
- Complications from Varicella
- People at High Hazard for Astringent Varicella
- Managing People at High Take a chance for Astringent Varicella
- Assessing Immunity to Varicella
- Preventing Varicella in Healthcare Settings
- Management of Patients with Varicella
- Impact of the Varicella Vaccination Program
Clinical Features
Incubation Catamenia and Prodrome
The average incubation period for varicella is 14 to 16 days after exposure to a varicella or a canker zoster rash, with a range of 10 to 21 days. A mild prodrome of fever and angst may occur i to 2 days earlier rash onset, particularly in adults. In children, the rash is ofttimes the outset sign of disease.
Varicella in Unvaccinated Persons
The rash is generalized and pruritic. Information technology progresses rapidly from macular to papular to vesicular lesions before crusting. Lesions are typically nowadays in all stages of evolution at the same time. The rash unremarkably appears first on the chest, back, and face, and then spreads over the entire torso. The lesions are commonly well-nigh concentrated on the chest and dorsum. Symptoms typically last 4 to 7 days.
In healthy children, varicella is by and large mild, with an itchy rash, malaise, and temperature up to 102°F for ii to three days. Infants, adolescents, adults, pregnant women, and immunocompromised people are at risk for more severe affliction and have a higher incidence of complications. Recovery from main varicella infection usually provides immunity for life. In otherwise healthy people, a 2nd occurrence of varicella is uncommon. Second occurrence of varicella may be more likely to occur in people who are immunocompromised. As with other viral infections, re-exposure to natural (wild-type) varicella may lead to re-infection that boosts antibody titers without causing illness or detectable viremia.
NEW: To learn more than almost how to promptly recognize and diagnose varicella, check out this Varicella Clinical Diagnosis Fact Sheet in English languagepdf icon and Spanishpdf icon .
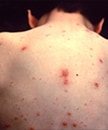
Characteristic pancorporeal varicella lesions in an unvaccinated person.
Varicella in Vaccinated Persons (Breakthrough Varicella)
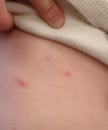
Breakthrough varicella on the abdomen of a vaccinated child.
Breakthrough varicella is infection with wild-type varicella-zoster virus (VZV) occurring in a vaccinated person more than 42 days afterwards varicella vaccination. Breakthrough varicella is unremarkably mild. Patients typically are afebrile or have low fever and develop fewer than l peel lesions. They usually have a shorter illness compared to unvaccinated people who get varicella. The rash is more likely to be predominantly maculopapular rather than vesicular. However, 25% to 30% of people vaccinated with one dose who get breakthrough varicella will take clinical features similar to unvaccinated people with varicella.
Since the clinical features of quantum varicella are often mild, it can be difficult to brand a diagnosis on clinical presentation solitary. Laboratory testing is increasingly of import for confirming varicella and accordingly managing the patients and their contacts. Breakthrough varicella occurs less frequently among those who have received two doses of vaccine compared with those who have received only one dose; disease may exist even milder among two-dose vaccine recipients, although the information about this is limited.
Transmission
Varicella is highly contagious. The virus tin be spread from person to person past direct contact, inhalation of aerosols from vesicular fluid of skin lesions of acute varicella or zoster, and maybe through infected respiratory secretions that also may exist aerosolized. A person with varicella is considered contagious kickoff 1 to two days before rash onset until all the chickenpox lesions have crusted. Vaccinated people may develop lesions that do not crust. These people are considered contagious until no new lesions have appeared for 24 hours.
It takes from ten to 21 days after exposure to the virus for someone to develop varicella. Based on studies of transmission amid household members, about 90% of susceptible close contacts volition get varicella subsequently exposure to a person with disease. Although limited information are available to assess the take chances of VZV manual from zoster, one household study constitute that the risk for VZV manual from canker zoster was approximately 20% of the risk for transmission from varicella.
People with quantum varicella are as well contagious. One study of varicella transmission in household settings found that people with mild quantum varicella (<50 lesions) who were vaccinated with one dose of varicella vaccine were ane-third equally contagious equally unvaccinated people with varicella. However, people with breakthrough varicella with fifty or more lesions were just as contagious as unvaccinated people with the disease.
Varicella is less contagious than measles, but more contagious than mumps and rubella.
Complications
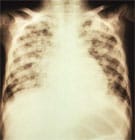
X-ray of pneumonia caused by varicella.
The well-nigh common complications from varicella are:
- In children: Bacterial infections of the skin and soft tissues
- In adults: Pneumonia
Severe complications caused past the virus include cerebellar ataxia, encephalitis, viral pneumonia, and hemorrhagic conditions. Other astringent complications are due to bacterial infections and include:
- Septicemia
- Toxic stupor syndrome
- Necrotizing fasciitis
- Osteomyelitis
- Bacterial pneumonia
- Septic arthritis
People at High Take a chance for Severe Varicella
People at take a chance for astringent varicella include:
- Immunocompromised people without evidence of amnesty to varicella, such equally:
- People with leukemia or lymphoma
- People on medications that suppress the immune system, such as loftier-dose systemic steroids or chemotherapeutic agents
- People with cellular immune-deficiencies or other allowed system problems
- Newborns whose mothers have varicella from five days earlier to 2 days after commitment
- Premature babies exposed to varicella or canker zoster, specifically:
- Hospitalized premature infants born at ≥28 weeks of gestation whose mothers practice not have evidence of amnesty
- Hospitalized premature infants built-in at <28 weeks of gestation or who weigh ≤one,000 grams at nascence regardless of their mothers' varicella amnesty status
- Significant women without evidence of immunity to varicella
Immunocompromised People
Immunocompromised people who become varicella are at risk of developing visceral dissemination (VZV infection of internal organs) leading to pneumonia, hepatitis, encephalitis, and disseminated intravascular coagulopathy. They tin can have an atypical varicella rash with more lesions, and they tin can be sick longer than immunocompetent people who get varicella. New lesions may continue to develop for more vii days, may announced on the palms and soles, and may be hemorrhagic.
People with HIV or AIDS
Children with HIV infection tend to have atypical rash with new crops of lesions presenting for weeks or months. The lesions may initially exist typical maculopapular vesicular but can afterward develop into non-healing ulcers that become necrotic, crusted, and hyperkeratotic. This is more probable to occur in HIV-infected children with depression CD4 counts.
Some studies accept plant that VZV dissemination to the visceral organs is less mutual in children with HIV than in other immunocompromised people with VZV infection. The rate of complications may also be lower in HIV-infected children on antiretroviral therapy or HIV-infected people with higher CD4 counts at the time of varicella infection. Retinitis tin occur among HIV-infected children and adolescents.
Most adults, including those who are HIV-positive, accept already had varicella and are VZV seropositive. Every bit a result, varicella is relatively uncommon among HIV-infected adults.
For more information nearly vaccinating immunocompromised people, including some groups with HIV-infection, see Special Considerations for Vaccination (Vaccination of HIV Infected Persons) in Prevention of Varicella: Recommendations of the Advisory Committee on Immunization Practices (ACIP).
Pregnant Women
Meaning women who go varicella are at risk for serious complications, primarily pneumonia, and in some cases, may dice as a effect of varicella. Some studies have suggested that both the frequency and severity of VZV pneumonia are higher when varicella is caused during the 3rd trimester, although other studies have not supported this observation.
If a significant adult female gets varicella in her start or early second trimester, her baby has a small risk (0.four to 2.0%) of being born with congenital varicella syndrome. The babe may have scarring on the skin; abnormalities in limbs, brain, and eyes, and low nascency weight.
If a woman develops varicella rash from five days before to 2 days after commitment, the newborn will be at hazard for neonatal varicella. Historically, the mortality rate for neonatal varicella was reported to exist about thirty%, merely the availability of VZV immune globulin and intensive supportive care take reduced the mortality to about 7%.
The vaccine is contraindicated for pregnant women. See Guidelines for Vaccinating Significant Women: Varicella.
Top of Folio
Managing People at High Run a risk for Severe Varicella
Varicella-Zoster Immune Globulin
For people exposed to varicella or herpes zoster who cannot receive varicella vaccine, varicella-zoster immune globulin can foreclose varicella from developing or lessen the severity of the disease. Varicella-zoster allowed globulin is recommended for people who cannot receive the vaccine and 1) who lack bear witness of immunity to varicella, 2) whose exposure is probable to result in infection, and iii) are at loftier adventure for severe varicella.
The varicella-zoster immune globulin product licensed for use in the United States is VariZIG™. VariZIG should exist given every bit before long equally possible after exposure to VZV; information technology tin can exist given within 10 days of exposure. For more than information on the recommendations for VariZIG employ, see the Morbidity and Mortality Weekly Report commodity on Updated Recommendations for Use of VariZIG — U.s., 2013. VariZIG is commercially bachelor from a broad network of specialty distributors in the United states of america (list bachelor at www.varizig.comexternal icon).
Acyclovir Treatment
The American Academy of Pediatrics (AAP) recommends that sure groups at increased risk for moderate to severe varicella exist considered for oral acyclovir or valacyclovir treatment. These high risk groups include:
- Healthy people older than 12 years of age
- People with chronic cutaneous or pulmonary disorders
- People receiving long-term salicylate therapy
- People receiving brusk, intermittent, or aerosolized courses of corticosteroids
Some healthcare providers may elect to use oral acyclovir or valacyclovir for secondary cases within a household. For maximum benefit, oral acyclovir or valacyclovir therapy should exist given within the first 24 hours after the varicella rash starts.
Oral acyclovir or valacyclovir therapy is not recommended by AAP for use in otherwise good for you children experiencing typical varicella without complications. Acyclovir is a category B drug based on the U.S. Food and Drug Administration'south Drug Risk Classification in pregnancy. Some experts recommend oral acyclovir or valacyclovir for pregnant women with varicella, specially during the second and third trimesters. Intravenous acyclovir is recommended for the pregnant patient with serious, viral-mediated complications of varicella, such as pneumonia.
Intravenous acyclovir therapy is recommended for astringent disease (e.g., disseminated VZV such equally pneumonia, encephalitis, thrombocytopenia, astringent hepatitis) and for varicella in immunocompromised patients (including patients existence treated with loftier-dose corticosteroid therapy for >14 days).
Famciclovir is available for treatment of VZV infections in adults, but its efficacy and safety accept not been established for children. In cases of infections caused by acyclovir-resistant VZV strains, which normally occur in immunocompromised people, Foscarnet should exist used to treat the VZV infection, but consultation with an infectious disease specialist is recommended.
Assessing Immunity to Varicella
Two doses of varicella vaccine are recommended for all children, adolescents, and adults without evidence of amnesty to varicella. Those who previously received i dose of varicella vaccine should receive their 2d dose for best protection confronting the disease.
Evidence of immunity to varicella includes any of the post-obit:
- Documentation of age-advisable varicella vaccination
- Preschool-historic period children (i.e., age 12 months through 3 years): one dose
- School-age children, adolescents, and adults: two doses
- Laboratory show of amnesty or laboratory confirmation of disease*
- Birth in the The states before 1980 (should not be considered bear witness of immunity for healthcare personnel, meaning women, and immunocompromised people)
- Diagnosis or verification of a history of varicella or herpes zoster past a healthcare provider
*Commercial assays can be used to assess disease-induced immunity, but they lack sensitivity to notice vaccine-induced immunity (i.e., they might yield simulated-negative results).
To verify a history of varicella, healthcare providers should inquire nigh:
- An epidemiologic link to another typical varicella example or to a laboratory confirmed case, or
- Evidence of laboratory confirmation, if testing was performed at the time of acute disease
People who take neither an epidemiologic link nor laboratory confirmation of varicella should not be considered every bit having a valid history of illness. For these people, a second dose of vaccine is recommended if they previously received simply one dose. If a healthcare provider verifies the diagnosis based on the above criteria, and then vaccination is not needed.
Routine testing for varicella amnesty after two doses of vaccine is not recommended. Bachelor commercial assays are non sensitive plenty to detect antibodies subsequently vaccination in all instances. Documented receipt of two doses of varicella vaccine supersedes results of subsequent serologic testing.
Preventing Varicella in Healthcare Settings
Nosocomial Transmission of VZV
Nosocomial transmission of VZV is well-recognized and can be life threatening to certain groups of patients. Reports of nosocomial transmission are uncommon in the U.s. since introduction of varicella vaccine.
Patients, healthcare providers, and visitors with varicella or herpes zoster can spread VZV to susceptible patients and healthcare providers in hospitals, long-term-intendance facilities, and other healthcare settings. In healthcare settings, transmissions have been attributed to delays in the diagnosis or reporting of varicella and canker zoster and failures to implement control measures promptly.
Although all susceptible patients in healthcare settings are at chance for severe varicella and complications, certain patients without evidence of immunity are at increased risk:
- Premature infants born to susceptible mothers
- Infants built-in at less than 28 weeks gestation or who weigh ≤m grams, regardless of maternal immune status
- Immunocompromised people, including those who are undergoing immunosuppressive therapy, have malignant disease, or are immunodeficient
- Significant women
Management of Patients with Varicella
Healthcare providers should follow standard precautions plus airborne precautions (negative air-period rooms) and contact precautions until lesions are dry and crusted. If negative air-flow rooms are not bachelor, patients with varicella should be isolated in closed rooms with no contact with people without show of immunity. Patients with varicella should exist cared for past staff with evidence of amnesty.
For more than information, run across:
- Preventing Varicella-Zoster Virus (VZV) Transmission from Zoster in Healthcare Settings
- Management of Patients with Herpes Zoster
- Management of Healthcare Personnel
- References and Resource
Impact of the Varicella Vaccination Program
Varicella used to exist very common in the Us. In the early 1990s, an boilerplate of 4 million people got varicella, 10,500 to thirteen,000 were hospitalized, and 100 to 150 died each yr.
Varicella vaccine became bachelor in the United states in 1995. Each twelvemonth, more than than 3.five million cases of varicella, 9,000 hospitalizations, and 100 deaths are prevented by varicella vaccination in the United States.
Highlights from Our Data
- Since introduction of the varicella vaccination programme in the United States, varicella morbidity (cases and hospitalizations) and mortality (deaths) has decreased past more than than 90%.
- Varicella incidence declined 98% during 1990-2016 in four states that consistently reported information to CDC. Varicella incidence, based on national passive surveillance data published in 2016, declined 85% betwixt 2005-2006 (before the two-dose recommendation) and 2013-2014, with the largest declines reported in children anile v-9 years (89.3%) and x-xiv years (84.8%).Varicella outbreaks have declined in size (i.e., number of cases) and duration.
- Varicella hospitalizations declined 93% in 2012 versus the pre-vaccine period; during the 2-dose varicella vaccination period (2006-2012), hospitalizations declined 38%.
- Varicella deaths declined overall by 94% during 2012-2016 compared with 1990-1994. In children and adolescents less than 20 years of age, varicella deaths declined by 99% during 2012-2016 compared with 1990-1994.
- Varicella incidence among infants – a group not eligible for varicella vaccination – declined past 90% from 1995 to 2008. Additionally, a decline in varicella incidence amongst HIV-infected children has been reported during the vaccination era.
- The charge per unit of canker zoster in United States amid children has been declining since the routine varicella vaccination programme began. National administrative data showed that canker zoster rates in people aged 0-17 declined 17% during 1993-2013.
Source: https://www.cdc.gov/chickenpox/hcp/index.html
0 Response to "Baby Chicken Pox From a Vaccinated Person Wild Strain or Not?"
Post a Comment